
Soda Ash The Second Largest End Use of Salt
Molecular Formula Na2CO3 CNa2O3 Synonyms SODIUM CARBONATE 497-19-8 Soda Ash Disodium carbonate Carbonic acid disodium salt View More. Molecular Weight 105.988 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Parent Compound CID 767 (Carbonic Acid) Component Compounds CID 5360545 (Sodium) CID 767 (Carbonic Acid) Dates Create: 2005-07-19

What Is the Difference between Baking Soda and Soda Ash? Uses & More
Sodium Carbonate is a generic term for Soda ash. Soda ash is made from Trona.. The chemical formula for Sodium Carbonate is Na 2 CO 3. When dissolved in water, this inorganic molecule produces carbonic acid and Sodium Hydroxide. In its purest state, it is a white powder with no odour. It's a strong base that can also be used as an antacid.

Bulk Soda Ash Chemical Formula Na2co3 Buy Soda Ash,Soda Ash Chemical
The chemical formula of soda ash is Na2CO3. The molar mass of anhydrous soda ash is roughly equal to 106 grams per mole. Under standard conditions for temperature and pressure (often abbreviated to STP), soda ash is known to exist as a white solid that is hygroscopic in nature. Is baking soda same as soda ash?

Soda Ash 10lbs Tie Dye Sodium Carbonate Washing Soda Stain
Let's have a look at some key differences between sodium carbonate and sodium bicarbonate. Sodium carbonate is often referred to as soda ash or washing soda. Sodium bicarbonate is popularly called as baking soda. . . Sodium carbonate is made up of sodium and acid. Sodium bicarbonate comes with sodium, acid and hydrogen.
PPT Analysis of Soda Ash PowerPoint Presentation, free download ID
Sodium carbonate, commonly known as soda ash in its anhydrous form, is the sodium salt of carbonic acid with the chemical formula Na 2 CO 3 . It occurs naturally in arid regions and is often extracted from mineral deposits of trona ore. Wyoming is the largest domestic source, with large natural trona deposits being mined.

PPT Analysis of Soda Ash PowerPoint Presentation, free download ID
The chemical formula of soda ash is Na 2 CO 3. The molar mass of anhydrous soda ash is roughly equal to 106 grams per mole. The decahydrate of this compound is known to have a molar mass of 286.14 grams per mole.

Soda Ash.docx Sodium Carbonate Chemical Substances Free 30day
It is carbonic acid's sodium salt with the chemical formula Na2CO3 N a 2 C O 3. It is frequently extracted from mineral resources of trona ore, where it naturally exists. The greatest domestic source, with sizable natural trona resources being extracted, is Wyoming.

Washing soda Acids, bases, and salts Chemistry Khan Academy YouTube
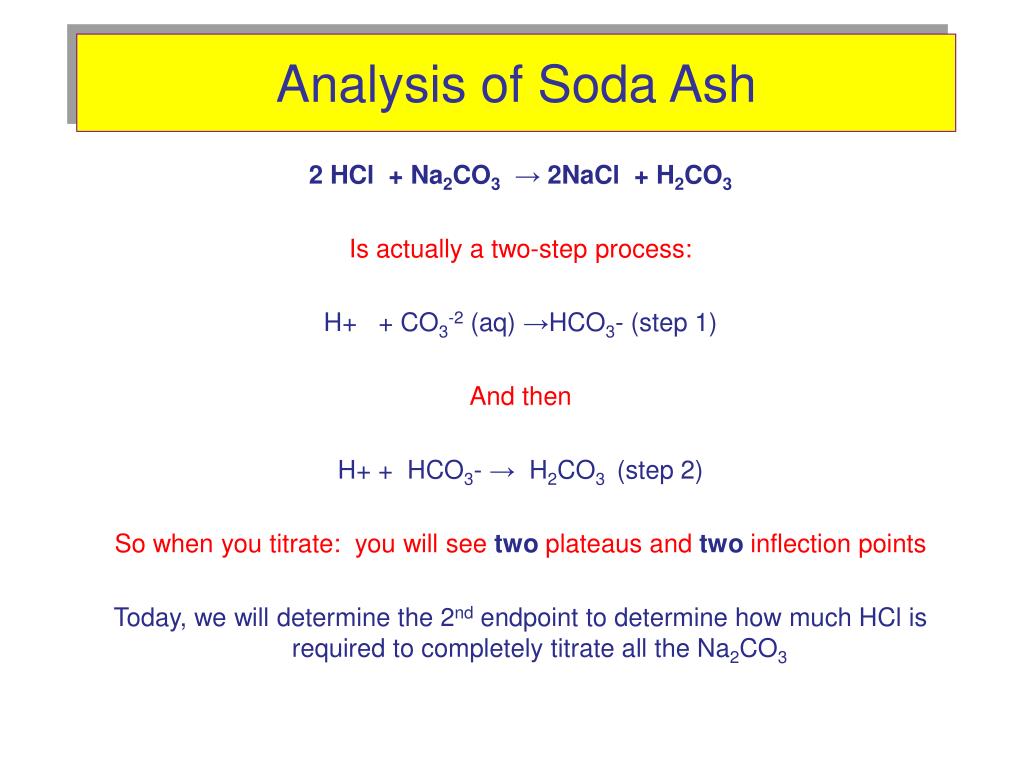
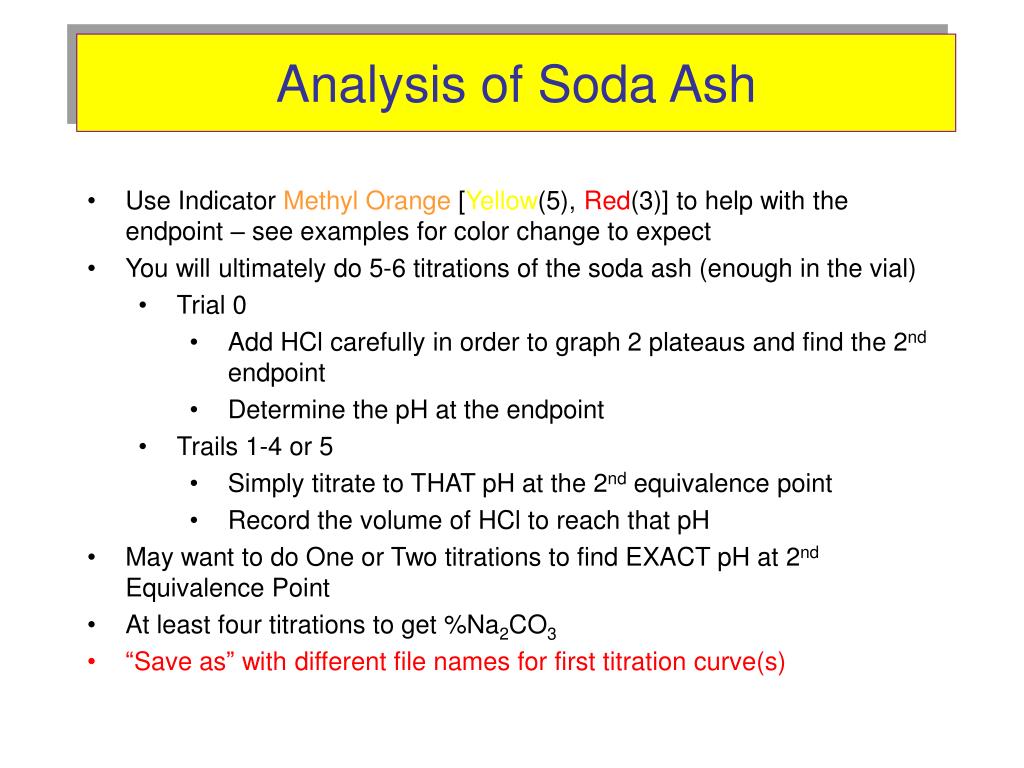
The titration involved in the determination of the carbonate content is an example of a weak base being titrated with a strong acid. The two weak bases involved are C O 3 2 − with K b 1 = 2.1 × 10 4 and H C O 3 − with K b 2 = 2.4 × 10 − 8. The reactions involved are: (1) C O 3 2 − + H 3 O + ⇌ H C O 3 − + H 2 O. and.

Soda Ash Complete Information Including Health Benefits, Selection
ABOUT SODA ASH. Soda ash, the trade name for sodium carbonate (Na2CO3), is a white, anhydrous, powdered or granular material. It is an essential raw material used in the manufacture of glass, detergents and soaps, chemicals and other industrial products. As such, soda ash is one of the most widely used and important commodities in the United.

Sodium Carbonate / Washing Soda / Soda Ash Chemical Formula China
soda ash, commonly known as sodium carbonate have chemical formula of… T4Tutorials.com Learn C programming, Data Structures tutorials, exercises, examples, programs, Database, Software, Data Mining, MCQs
Sodium carbonate, Na2CO3, natrium carbonate, washing soda, soda ash
Sodium carbonate is a diazonium salt of carbonic acid with the chemical formula Na2CO3. It is also known as Soda crystals, soda ash, washing soda. This inorganic compound is water-soluble and when dissolved in water, it forms carbonic acid and sodium hydroxide. In its pure form, it is a white powder and odourless.
PPT Analysis of Soda Ash PowerPoint Presentation, free download ID
Solvay process The Solvay process or ammonia-soda process is the major industrial process for the production of sodium carbonate (soda ash, Na 2 CO 3 ). The ammonia-soda process was developed into its modern form by the Belgian chemist Ernest Solvay during the 1860s. [1]

Soda Ash Dense Sodium Carbonate Able Westchem
Sodium carbonate. Sodium carbonate (also known as washing soda or soda ash ), Na 2 CO 3, is a sodium salt of carbonic acid. It most commonly occurs as a crystalline heptahydrate, which readily effloresces to form a white powder, the monohydrate. It has a cooling alkaline taste, and can be extracted from the ashes of many plants.
Sodium Carbonate, Na2CO3, Natrium Carbonate, Washing Soda, Soda Ash
Sodium carbonate, also known as soda ash or washing soda, is a chemical compound with the formula Na2CO3. It is a white, crystalline solid that is commonly used in various industries. Sodium carbonate has a wide range of applications, including as a cleaning agent, pH regulator, and water softener.It is also used in the production of glass, paper, and textiles.

Soda ash chemical formula is
Soda Ash is the 10th most consumed inorganic compound in the world, which has been used for over 5,000 years. It is a safe, simple compound and a key component in a variety of industrial processes from the manufacture of glass to dry powder detergents and lithium-ion batteries. It is also an important ingredient in the food and pharmaceutical.

Soda Ash
Soda Ash (Sodium carbonate), Na 2 CO 3 .10H 2 O, is an industrial chemical with the formula Na2CO3. It is also known as washing soda, soda ash, and soda crystals. All of the forms are water-soluble, colorless, odorless salts that produce moderately alkaline solutions in water. Soda Ash What is Soda Ash (Sodium Carbonate) used for?